Keeping steel structures safe from corrosion is difficult, but traditional coatings contain chemicals that harm aquatic life. Bernhard Münzing explains how graphene could offer a more eco-friendly solution

For as long as steel structures have existed, the people who maintain them have had to worry about corrosion. According to the World Corrosion Organization, corrosion causes $2.5tr in damage to steel structures every year – approximately 3–4% of the annual GDP of industrialized countries. Traditionally, there have been several ways of reducing this damage and its associated costs, including galvanization with zinc, chromium and other metals. In highly corrosive environments, zinc-rich primers are often used, while in medium- to low-corrosion areas, primers with passive corrosion pigments such as phosphates may be sufficient. In both cases, the primer is followed by medium and top coats of paint.
Recently, new primers have been introduced that contain zinc along with additional pigments. These primers aim to fulfil the latest requirements for corrosion protection, which are set out in an international standards document (ISO 12944-2018). Unfortunately, zinc products, such as the commonly used zinc powder, are highly toxic to aquatic life. Users in marine environments are therefore increasingly demanding primers with a much reduced zinc content.
This is where graphene, the monolayer form of graphite, comes into play. This material was first detected in 2004, and its exceptional mechanical strength, along with its excellent electrical and thermal conductive properties, make it attractive for a range of applications. Graphene can also absorb atoms or molecules and can be functionalized by bonding different chemical groups to its carbon atoms.
Over the past 15 years, scientists and engineers have established several routes for producing graphene industrially. For applications in the electronics industry, chemical vapour disposition (CVD), which starts with a carbon-rich atmosphere and deposits a single layer of carbon atoms onto a substrate, is normally used to create graphene sheets with a high electrical conductivity. Another common route is to use a modified Hummers method, in which graphite is first oxidized, and then, through reduction steps carried out in an inert atmosphere, different graphene types are produced. Other methods include peeling off, or exfoliating, layers of graphene using a proprietary electrochemical process.
With the exception of CVD, these methods tend to produce few-layer graphene products, which are available either as a powder or dispersed in solvents, water and polymer systems. The primary particles of a few-layer graphene product might have lateral sizes of 1 µm to more than 50 µm, with a thickness of up to a few nanometers, depending on the number of layers. Even though these products are not pure graphene, their electrical and thermal conductivity and their mechanical properties are very similar to those of the pure material. Crucially, they are close enough for corrosion-protection purposes.
Corrosion, in the most common use of the word, is the electrochemical oxidation of metal (usually steel) with an oxidant such as oxygen, sulphates or chlorides to form chemically stable metal salts – that is, rust. Being a conductive material, graphene is able to influence the electrochemical reaction (together with the second complementary anti-corrosion pigment) in a favourable way, meaning less rust. The barrier properties of graphene support this effect. Additionally, graphene can strengthen the adhesion of the binder in the coating system to the substrates. This helps to prevent the (salty) water, which attacks the substrates, from separating the protective coating from the substrate.
Environmental protection
In 2012 the Chinese government issued a mandate for reducing the zinc content in zinc-rich primers, with the aim of reducing zinc oxide leaching and thus protecting the environment during the lifetime of the primers. In response, scientists at The Sixth Element began to evaluate the potential of graphene as an additional pigment in corrosion-protection coatings. By mid-2015 we had developed a 2K epoxy system – the “2K” here is industry shorthand indicating a two-component coating – containing graphene and a lower amount of zinc powder. A patent for this system has been granted in China and the US.
While we were developing our zinc-based, graphene-enhanced corrosion protection primer, our researchers made a few very important observations. One is that the graphene powder must be very well dispersed and de-agglomerated throughout the primer, because only the primary particles of graphene are responsible for its outstanding properties. Another is that adding graphene to a coating on its own will actually speed up corrosion, because steel is less “noble” (it reacts more readily) than carbon. Only when graphene is combined with other corrosion-protection pigments is it able to perform an anti-corrosion function. Under these conditions, our experiments showed that graphene acts as very good barrier against both oxidation and chemical attack from immersion in salty, acidic water. We believe this is because, for a given dry-film thickness, the number of graphene particles (with a lateral size of a few microns and a thickness of no more than a few nanometres) is much higher compared to that of standard barrier pigments, which have a spherical particle size of 10 µm and up. Essentially, salty water has to find a way around the primary particles before it can finally come into contact with the surface of the substrate. The rate of immersion is very much reduced.
We also learned that the degree of corrosion resistance for a given coating depends on the amount of both the graphene and the second, synergistic, corrosion-protection pigment. The optimum amounts differ for each second pigment used, and finding them has to be done experimentally, because so far no general theory has been established. We do, however, know that when the second pigment is zinc powder, graphene acts to strengthen the conductive network between the zinc particles. This means that the corrosion-induced current can dissipate much more easily from the surface. Additionally, graphene is able to “donate” electrons from its sp2 orbitals either to zinc or to the protons in acidic water, both of which make it harder for acidic components to attack the steel surface below the coating.
During the development of the primer system, questions were raised about whether graphene would harm the adhesion of further coatings applied on top of the primer. Extensive testing showed that this is not the case, provided the mechanical properties of the substrate, the primer and further coatings are well matched. The adhesion of further coatings to the primer very much depends on the resin system used and the general rules established for the different resin systems apply. In fact, graphene may actually enhance the adhesion of the coating to the substrate, because it acts as a reinforcing material in resin systems (resulting in a higher elastic modulus). The better the resin is adjusted to the substrate and the prevailing temperature/humidity conditions, the lower the amount of graphene that can be added without jeopardizing the adhesion of the coating to the substrate.

The right formulation
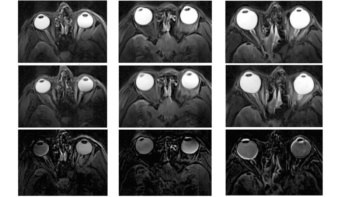
Using these observations, developers at The Sixth Element have produced a 2K epoxy primer formulation (containing 25% zinc powder and 1% graphene by dry-film weight) that fulfils all requirements for highly corrosive environments, if the appropriate medium and top coating are applied. In standard salt-spray testing (using 50 ± 5 g of sodium chloride per litre of water at 35 ± 2 °C), the primer alone, with a dry-film thickness of 50 µm, could withstand more than 3000 hours in this harsh environment (see image above).
Before using this primer in a real application, independent institutes – in this case in China – tested it, along with the proposed medium and top coats (which are necessary to achieve the dry-film thickness as required by industry standards), under different conditions. Salt-water spray testing and condensation testing at these institutes confirmed that the norms valid at the time of testing have been fulfilled, so the primer was approved for use in heavy-corrosion situations such as marine environments.
The system we developed got its first “real world” test in 2015, when it was applied to the steel components of a wind farm. As this technology was then completely new, The Sixth Element and our commercial partners (a coating company that produced the graphene-enhanced coating to our specifications) had to deposit €1m as a security, just in case – for whatever reason – the coating failed, necessitating expensive repairs. After two years and several detailed inspections, we got our deposit back.
Only when graphene is combined with other corrosion-protection pigments is it able to perform an anti-corrosion function
Soon afterwards, we began testing modifications to our formula, with the aim of matching customers’ price/performance expectations. One such modification produced a solvent-based 2K epoxy system with 37.5% zinc powder and 0.35% graphene (all based on dry film) that also achieved the performance requirements set by the Chinese authorities. In another experiment, a water-based 2K epoxy system formulated with 45% zinc powder and 1% graphene showed exceptional corrosion-protection properties, withstanding 2400 hours in standard salt spray testing. However, as these examples show, there is not a linear correlation between the amounts of graphene and second corrosion-protection pigment. As no theory has been established for predicting the right mixtures, only experiments will lead to good results.
Since these initial tests were carried out, several companies have established graphene-based corrosion protection products on the market. As of December 2018, they included China’s Topsen (the first in the world); a small Greek company called Hydroton; and James Brigg, which manufactures a graphene product for the consumer market under the Hycote brand.
Future applications
After gaining experience of using graphene in conjunction with zinc powder, companies have now begun to evaluate the potential of combining graphene with phosphates and other passive corrosion-protection pigments, with an eye towards reducing zinc usage still further. In these cases, the barrier function of graphene is expected to be the dominant protection mechanism. However, if acidic water is present, graphene may also support chemical reactions between the passive corrosion-protection pigments and the metal surface. The theory is that faster reactions of this type would mean that iron salts (in most cases phosphate) form more quickly, so that the pure iron spends less time exposed to the acidic environment.
The new standards for marine environments pose a big challenge for all coating companies. Under ISO 12944-2018, manufacturers are now obligated to run several 4200-hour tests of how coatings behave under different temperatures, humidity and immersion levels. A material must pass all these tests before it qualifies for use in marine environments. We believe that graphene can play a vital role in fulfilling these requirements, or even going beyond them – thus producing better corrosion resistance at lower environmental cost.
- Enjoy the rest of the 2019 Physics World Focus on Nanotechnology & Nanomaterials in our digital magazine or via the Physics World app for any iOS or Android smartphone or tablet.